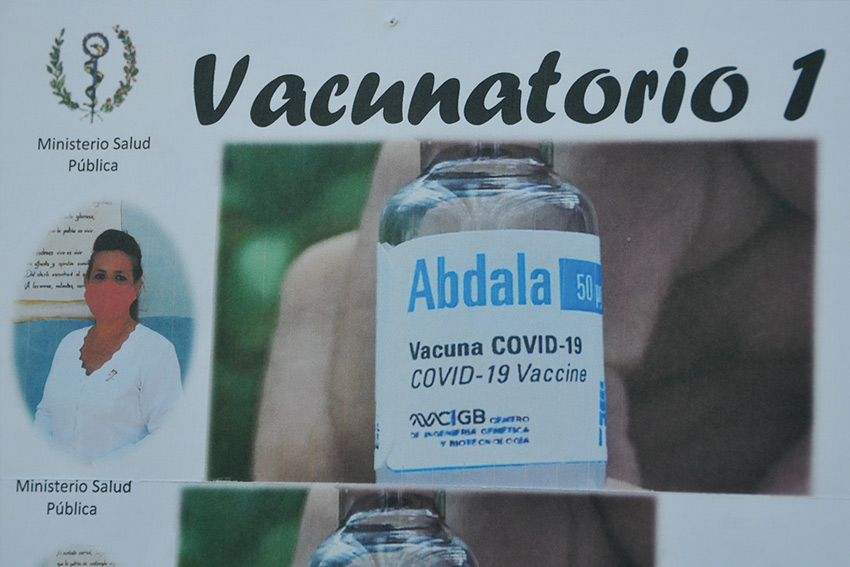
The New Molecules Committee (CMN) of the Federal Commission for Protection against Sanitary Risks (COFEPRIS) issued today a favorable opinion on the Cuban recombinant protein vaccine Abdala.
Mexico City.- The drug, developed by Cuba's Center for Genetic Engineering and Biotechnology (CIGB), is indicated for specific active immunization against SARS-CoV-2 infection in children aged five years and older, with efficacy against mild, moderate, and severe clinical forms of Covid-19 of 92.13 percent, 88.99 percent and 92.33 percent, respectively, according to the CMN.
The opinion issued is part of the process to obtain emergency use authorization issued by the Cofepris Health Authorization Commission, once the information submitted has been evaluated.
In a statement, Cofepris explained that on April 3, the Abdala vaccine obtained, from the National Committee of Science, Technology, and Innovation in Public Health (CNCTI-SP), of the now National Council of Humanities, Sciences, and Technologies, a favorable recommendation for the extension of the indication for emergency use for primary vaccination in the pediatric population from 5 to 17 years of age.
Since 2021, the Center for State Control of Medicines, Equipment and Medical Devices (CECMED) of Cuba, authorized the emergency use of that vaccine in that country, by confirming that it met the requirements and parameters demanded in terms of quality, safety, and efficacy.
This health authority reminds the population that the supply of vaccines to prevent the pandemic is universal and free of charge and that in the country its application is established in the National Vaccination Policy against the SARS-CoV-2 virus, for the Prevention of COVID-19 in Mexico. (PL)